Primary and secondary cells
primary cells
Some electrical cells, once their potential (chemical) energy has all been changed to electricity and used up, must be thrown away. They are no good anymore.
These are called primary cells.
Primary cells include the ones you usually put in a flashlight, in a transistor radio, and in various other consumer devices.
They use dry electrolyte pastes along with metal electrodes.
They go by names such as dry cell, zinc-carbon cell, alkaline cell, and others.
If we go into a department store and find the panel of batteries, and we’ll see
various sizes and types of primary cells, such as AAA batteries, D batteries, camera batteries, and watch batteries.
You should know by now that these things are cells, not true batteries.
You’ll also see real batteries, such as the little 9-V transistor batteries and the large 6-V lantern batteries.
secondary cells
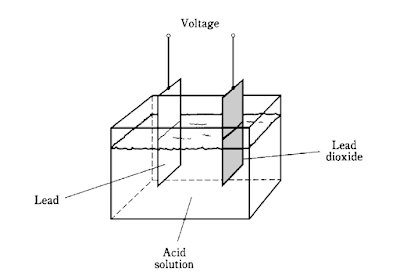
Other kinds of cells, like the lead-and-acid unit depicted above, can get their chemical
energy back again. Such a cell is a secondary cell.
Secondary cells can also be found increasingly in consumer stores.
Nickel-cadmium (Ni-Cd or NICAD) cells are probably the most common.
They’re available in some of the same sizes as nonrechargeable dry cells.
The most common sizes are AA, C, and D. These cost several times as much as ordinary dry cells, and a charging unit.
But if you take care of them, these rechargeable cells can be used hundreds of times and will pay for themselves several times over if you use a lot of “batteries” in your everyday life.
The battery in your car is made from secondary cells connected in series.
These cells recharge from the alternator or from an outside charging unit.
This battery has cells likethe one in Fig.
. It is extremely dangerous to short-circuit the terminals of such a battery,
because the acid (sulfuric acid) can “boil” out and burn your skin and eyes.
Never short-circuit any cell or battery
An important note is worth making here: Never short-circuit any cell or battery, because
it might burst or explode.
 |
| Lead acid battery |
- Grouping of Cells|Series, parallel and Mixed Cell combinations
- MCQ’s on Cells and Batteries|Cells and batteries Objective Questions
- Cells and batteries
- Basic Electrical Engineering Quiz | Cells and Batteries Quiz
- Cells and batteries
- Primary and secondary cells|Cells and Batteries
- Battery Capacity Tester|Battery Tester