ONE OF THE MOST COMMON AND MOST VERSATILE SOURCES OF DC IS THE CELL.
The term cell means self-contained compartment, and it can refer to any of various different things in (and out of) science.
Electrical Cell
In electricity and electronics, a cell is a unit source of dc energy.
There are dozens of different types of electrical cells.
When two or more cells are connected in series, the result is known as a battery.
Kinetic and potential energy
Energy can exist in either of two main forms.
Kinetic energy
Kinetic energy is the kind you probably think of right away when you imagine energy.
A person running, a car moving down a freeway, a speeding aircraft, a chamber of superheated gasall these things are visible manifestations of kinetic energy, or energy in action.
The dissipation of electrical power, over time, is a form of kinetic energy too.
Potential energy
Potential energy is not as vividly apparent.
When you raise a block of concrete into the air, you are creating potential energy.
You remember the units called foot-pounds, the best way to measure such energy, from school physics classes.
If you raise aone-pound weight a foot, it gains one foot pound of potential energy. If you raise it 100feet, it gains 100 foot pounds. If you raise a 100-pound weight 100 feet, it will gain 100× 100, or 10,000, foot pounds of potential energy.
This energy becomes spectacularly evident if you happen to drop a 100-pound weight from a tenth-story window.
Electrochemical energy
In electricity, one important form of potential energy exists in the atoms and molecules of some chemicals under special conditions.
Early in the history of electrical science, laboratory physicists found that when metals came into contact with certain chemical solutions, voltages appeared between the pieces of metal.
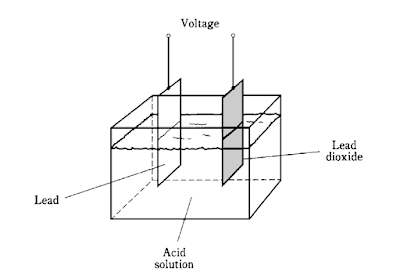
A piece of lead and a piece of lead dioxide immersed in an acid solution (Fig.)will show a persistent voltage.
This can be detected by connecting a galvanometer between the pieces of metal.
A resistor of about 1,000 ohms should always be used in series with the galvanometer in experiments of this kind; connecting the galvanometer directly will cause too much current to flow, possibly damaging the galvanometer and causing the acid to “boil.”
The chemicals and the metal have an inherent ability to produce a constant exchange of charge carriers.
If the galvanometer and resistor are left hooked up between the two pieces of metal for a long time, the current will gradually decrease, and the electrodes will become coated.
The acid will change, also.
The chemical energy, a form of potential energy in the acid, will run out.
All of the potential energy in the acid will have been turned into kinetic electrical energy as current in the wire and galvanometer.
In turn, this current will have heated the resistor (another form of kinetic energy), and escaped into the air and into space.
- Grouping of Cells|Series, parallel and Mixed Cell combinations
- MCQ’s on Cells and Batteries|Cells and batteries Objective Questions
- Cells and batteries
- Basic Electrical Engineering Quiz | Cells and Batteries Quiz
- Cells and batteries
- Primary and secondary cells|Cells and Batteries
- Battery Capacity Tester|Battery Tester